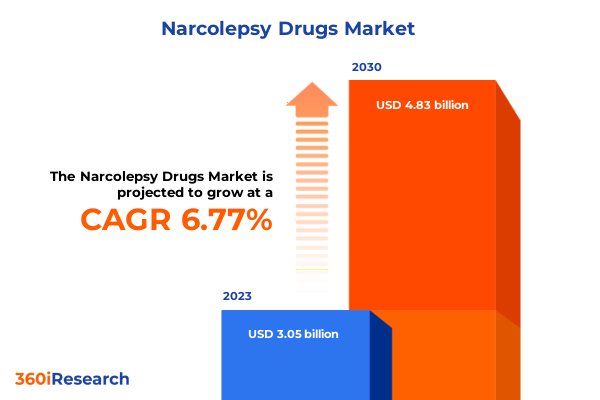
The Narcolepsy Drugs Market size was estimated at USD 3.28 billion in 2024 and expected to reach USD 3.51 billion in 2025, at a CAGR 6.81% to reach USD 5.57 billion by 2032.
Unraveling the Complexities of Narcolepsy Therapeutics in a Rapidly Evolving Scientific and Regulatory Environment Driving Patient Outcomes and Market Dynamics
Narcolepsy presents a complex clinical challenge that combines chronic neurological dysfunction with profound impacts on patient quality of life. With excessive daytime sleepiness, cataplexy episodes, and disrupted nocturnal sleep defining the disorder, healthcare systems and pharmaceutical innovators have prioritized effective therapeutic management. In recent years, the field has shifted from general stimulant-based interventions toward targeted modulators of wakefulness pathways. Such a transformation underscores an urgency for stakeholders to reassess existing care paradigms in light of emerging mechanisms of action and evolving regulatory criteria.
Against this backdrop, our executive summary examines the pivotal developments shaping narcolepsy drug landscapes. By synthesizing advances in drug formulation, distribution models, and patient access strategies, the following report distills key drivers of change. It also highlights structural pressures-ranging from tariff-induced supply chain realignments to regional reimbursement nuances-that require proactive planning. Through an integrated lens, decision-makers will better appreciate how scientific breakthroughs, policy shifts, and competitive tactics converge to define market trajectories. Ultimately, this introduction sets the stage for a comprehensive exploration of how innovation and regulation jointly propel the next chapter in narcolepsy care.
Disruptive Innovations and Breakthrough Approvals Redefining Treatment Paradigms and Clinical Management Strategies in Narcolepsy Care
The past five years have witnessed a confluence of scientific and regulatory events that have fundamentally altered treatment paradigms in narcolepsy. For instance, the approval of novel orexin receptor agonists has introduced a mechanism distinct from traditional psychostimulants, opening new avenues for clinical efficacy and tolerability. Concurrently, sodium oxybate formulations have evolved, with once-nightly extended-release options promising enhanced patient adherence and reduced nocturnal awakenings. These shifts have catalyzed a broader reassessment of long-standing clinical guidelines and created pathways for more personalized therapeutic regimens.
Moreover, regulatory bodies have begun to streamline approval processes for neurotherapeutics, reflecting an increased willingness to accommodate breakthrough status designations. This has accelerated the transition from late-stage clinical trials to market availability, compressing launch timelines and intensifying competitive pressures. At the same time, telemedicine platforms have proliferated, enabling sleep specialists to integrate digital patient monitoring and remote titration protocols. As such, the intersection of novel pharmacology, regulatory agility, and digital health represents a transformative wave that stakeholders must navigate to capture emerging value.
Economic Pressures from 2025 Tariff Policies Shaping Supply Chain Resilience and Cost Structures in Narcolepsy Drug Development and Distribution
In 2025, the United States implemented targeted tariff measures on select pharmaceutical raw materials and chemical intermediates, aiming to bolster domestic manufacturing capacity. While these policies were designed to reduce reliance on offshore suppliers, they have cumulatively increased input costs for active pharmaceutical ingredients linked to narcolepsy treatments. Factories sourcing precursors from traditional hubs have faced 10 to 15 percent cost uplifts, prompting many to reassess supplier contracts and consider near-shoring options to mitigate margin erosion.
Simultaneously, the broader impact of these tariffs has manifested in supply chain recalibrations and inventory realignments. Manufacturers have accelerated qualification processes for secondary sourcing in North America, leading to greater redundancy in API supply routes but also elevating complexity in quality oversight. These dynamics have compelled partnerships between pharma players and specialized contract development and manufacturing organizations to ensure consistent production volumes. As a result, cost structures for both branded and generic narcolepsy medications now incorporate new layers of logistical and compliance overhead, reinforcing the need for integrated risk management strategies across the value chain.
Deconstructing Market Segmentation Dynamics Across Diverse Drug Classes Formulations Distribution Channels and End User Profiles in Narcolepsy Care
Segmenting the narcolepsy therapeutics space reveals nuanced insights into where value and opportunity concentrate. In examining drug classes, amphetamine salts have maintained a legacy role but face erosion from the modafinil derivatives category, which includes armodafinil and modafinil. Meanwhile, novel agents such as pitolisant and solriamfetol have carved out niches by targeting histaminergic and dopaminergic pathways respectively, challenging sodium oxybate’s dominance in remediating cataplexy and disruptive nocturnal sleep. Product positioning within these classes hinges on differentiating efficacy profiles and side-effect tolerability.
Distribution channels further shape commercial access, with hospital pharmacies serving as primary conduits for initiation and dose titration, while retail pharmacies and online fulfillment models optimize long-term patient convenience. Formulation preferences also impact adherence; capsules and tablets predominate for their familiarity and ease of dosing, whereas liquid solutions cater to specialized populations requiring titration flexibility or pediatric administration. Finally, end users span home environments, where self-administration imparts autonomy, to hospitals and sleep clinics, where expert oversight supports complex titration and monitoring. By synthesizing trends across these segments, stakeholders can prioritize which portfolios, channels, and end-user engagements will most effectively drive adoption and patient satisfaction.
This comprehensive research report categorizes the Narcolepsy Drugs market into clearly defined segments, providing a detailed analysis of emerging trends and precise revenue forecasts to support strategic decision-making.
- Drug Class
- 流通チャネル
- Formulation
- エンドユーザー
Unveiling Region-Specific Trends Impacting Accessibility Reimbursement and Patient Adoption Rates Across Americas EMEA and Asia-Pacific Markets
Regional analysis of narcolepsy therapeutics underscores significant variation in treatment access and adoption. In the Americas, reimbursement frameworks and established patient registries facilitate expedited coverage of both legacy stimulants and newer wakefulness agents. Country-level policies incentivize local manufacturing investments, reinforcing supply stability amid evolving tariff environments. Meanwhile, the Europe, Middle East & Africa region grapples with heterogeneous regulatory landscapes; Western European markets often mirror U.S. approval timelines, whereas emerging EMEA territories encounter delayed launches due to pricing negotiations and reimbursement reviews. This has fostered parallel import strategies and regional harmonization initiatives.
Across Asia-Pacific, rising healthcare expenditure and growing awareness of sleep disorders have accelerated market expansion. Regulatory agencies in Japan and Australia are increasingly open to novel mechanisms of action, shortening review cycles for breakthrough therapies. However, in markets such as China and India, price controls and volume-based procurement exercises exert considerable downward pressure on average selling prices. In response, global and regional manufacturers are forging strategic alliances with local distributors and contract partners to navigate market access complexities. Consequently, tailored go-to-market strategies become indispensable for capturing growth potential within each geographic cluster.
This comprehensive research report examines key regions that drive the evolution of the Narcolepsy Drugs market, offering deep insights into regional trends, growth factors, and industry developments that are influencing market performance.
- 米州
- ヨーロッパ、中東、アフリカ
- アジア太平洋
Strategic Movements and Competitive Positioning of Leading Biopharmaceutical and Generic Innovators Driving the Narcolepsy Drug Sector Forward
Leading companies in narcolepsy therapeutics are actively repositioning portfolios through strategic acquisitions, partnerships, and pipeline diversification. Jazz Pharmaceuticals continues to strengthen its sodium oxybate franchise through lifecycle extension programs and formulation refinements, while simultaneously exploring orexin modulator candidates via research collaborations. Axsome Therapeutics has achieved traction with solriamfetol by securing label expansions in multiple jurisdictions, leveraging pharmacoeconomic data to support favorable reimbursement outcomes.
Meanwhile, manufacturers of modafinil derivatives, including Teva Pharmaceuticals, face looming patent expirations and are proactively developing next-generation analogs to maintain competitive differentiation. Harmony Biosciences has made inroads with pitolisant in key European markets by deploying targeted physician engagement campaigns and real-world evidence studies. Generic entrants are also reshaping competitive dynamics, focusing on cost leadership and specialty pharmacy partnerships to capture share among established treatment regimens. Across the board, corporate strategies emphasize broadening therapeutic pipelines, enhancing patient support services, and fortifying manufacturing resilience to navigate evolving regulatory and tariff pressures.
This comprehensive research report delivers an in-depth overview of the principal market players in the Narcolepsy Drugs market, evaluating their market share, strategic initiatives, and competitive positioning to illuminate the factors shaping the competitive landscape.
- Jazz Pharmaceuticals plc
- Harmony Biosciences Holdings, Inc.
- Avadel Pharmaceuticals plc
- テバ・ファーマシューティカル・インダストリーズ社
- Viatris Inc.
- Hikma Pharmaceuticals PLC
- Sandoz Inc.
- Lupin Limited
- Cipla Limited
- Glenmark Pharmaceuticals Ltd.
Actionable Strategic Priorities to Enhance Research Innovation Optimize Supply Chains and Strengthen Stakeholder Engagement in Narcolepsy Treatment
Industry leaders should prioritize a multifaceted strategic agenda to capitalize on emerging narcolepsy treatment opportunities. First, diversifying API sourcing by establishing dual-supplier arrangements in both traditional and near-shore locales will mitigate tariff-related risks and sustain production volumes. Simultaneously, investing in digital health platforms that facilitate remote patient monitoring and dose optimization can bolster adherence while reducing clinical burden. Linking these platforms to pharmacovigilance frameworks ensures real-time safety oversight and enriches post-marketing data.
In parallel, companies must engage payers through robust health economics and outcomes research, demonstrating the long-term value of novel mechanisms relative to legacy treatments. Coordinating with regulatory authorities to secure breakthrough designations and accelerated review pathways will also be essential for compressing time-to-market. Finally, cultivating partnerships with sleep clinics and patient advocacy groups can amplify disease awareness, support patient education, and expand recruitment channels for clinical trials. By integrating supply chain resilience, digital innovation, payer alignment, and stakeholder collaboration, firms can establish sustainable competitive advantages in narcolepsy care.
Comprehensive Multi-Source Research Approach Employing Primary Interviews Secondary Research and Rigorous Data Triangulation for Report Integrity
This report employs a rigorous research framework combining primary and secondary methodologies to ensure comprehensive and validated insights. Primary research consisted of in-depth interviews with key opinion leaders, including neurologists, sleep specialists, and pharmacoeconomists, to capture qualitative perspectives on emerging therapies and market access challenges. These interviews informed hypothesis generation and guided subsequent analytical phases. Secondary research involved systematic desk analysis of regulatory filings, clinical trial registries, government policy documents, and peer-reviewed literature to establish contextual benchmarks and trend trajectories.
Data triangulation underpins the report’s accuracy, integrating quantitative inputs from prescription databases, hospital pharmacy audits, and distribution channel metrics. Segmentation analyses across drug class, distribution channel, formulation, and end user were performed to identify high-impact subsegments. Regional insights derive from country-level reimbursement schedules, tariff notifications, and local market intelligence. Lastly, an iterative review process with a panel of advisory experts ensured alignment with industry developments and validated the interpretive synthesis of findings.
This section provides a structured overview of the report, outlining key chapters and topics covered for easy reference in our Narcolepsy Drugs market comprehensive research report.
- 序文
- 研究方法
- エグゼクティブ・サマリー
- 市場概要
- 市場インサイト
- 米国関税の累積影響 2025年
- 人工知能の累積インパクト 2025年
- Narcolepsy Drugs Market, by Drug Class
- Narcolepsy Drugs Market, by Distribution Channel
- Narcolepsy Drugs Market, by Formulation
- Narcolepsy Drugs Market, by End User
- Narcolepsy Drugs Market, by Region
- Narcolepsy Drugs Market, by Group
- Narcolepsy Drugs Market, by Country
- 競争環境
- 図リスト【計28
- List of Tables [Total: 502 ]
Concluding Insights Emphasizing Strategic Agility and Collaborative Innovation as Pillars for Sustained Progress in Narcolepsy Therapy Development
In conclusion, the narcolepsy therapeutics landscape stands at an inflection point where scientific breakthroughs, policy reforms, and supply chain realignments converge. The advent of orexin receptor agonists and extended-release formulations signals a shift toward more precise and patient-centric interventions. At the same time, tariff-driven cost pressures and regional reimbursement divergences demand agile responses in sourcing, pricing, and market entry strategies.
By deconstructing segmentation dynamics and benchmarking regional performance, stakeholders can pinpoint areas of unmet need and growth potential. Strategic maneuvers by leading innovators underscore the importance of portfolio diversification, digital integration, and payer engagement. Moving forward, the industry must sustain momentum through collaborative research initiatives, adaptive supply chain frameworks, and proactive regulatory dialogues. Embracing these imperatives will not only elevate patient outcomes but also secure sustainable value creation within the evolving narcolepsy drug sector.
Engage with Ketan Rohom to Unlock Exclusive Insights and Secure Your Comprehensive Narcolepsy Therapeutics Market Research Report Today
To explore how these insights apply to your organization and to secure a comprehensive analysis of global narcolepsy therapeutics trends, reach out directly to Ketan Rohom, Associate Director, Sales & Marketing at 360iResearch. Engage in a personalized consultation to discuss how the findings align with your strategic objectives and gain access to proprietary data visualizations, executive briefings, and in-depth company profiles. Seize this opportunity to inform your next product strategy, partnership negotiation, or investment decision with confidence and precision

- How big is the Narcolepsy Drugs Market?
- What is the Narcolepsy Drugs Market growth?
- 報告書はいつもらえますか?
- この報告書はどのような形式で送られてくるのですか?
- 360iResearchはいつからあるのですか?
- レポートについて質問がある場合は?
- このレポートをチームで共有できますか?
- あなたの研究をプレゼンテーションに使ってもいいですか?




